Participate in the Clinical Advancement of Reproductive Health
Gestational and gamete donation represents a significant contribution to the field of Assisted Reproductive Technology (ART). By participating as an egg donor with Uzazi Mwelekeo Agency, you provide essential clinical support to individuals and couples navigating complex infertility challenges.
Our program is built upon a foundation of participant welfare and informed consent. We prioritize the health and safety of our donors through rigorous medical oversight and structured psychosocial support, ensuring that your participation is ethically governed and clinically managed at the Dr. Garang IVF and Endoscopy Institute.
The Impact of Clinical Oocyte Donation
Participating in an egg donation program is a significant contribution to the field of Assisted Reproductive Technology (ART). Your participation supports clinical solutions for individuals navigating complex reproductive challenges.
- Commitment and Participant Welfare:
- Advancement of Fertility Solutions: Provide the essential biological foundation for evidence-based fertility treatments.
- Professional Welfare Support: Experience the professional satisfaction of supporting reproductive health outcomes within a bioethically governed framework
- Stipendiary Reimbursement: Receive structured financial reimbursement for the time, travel, and logistical commitments required throughout the clinical cycle.
- Rigorous Medical Oversight: Undergo a high-standard diagnostic and monitoring process at the Dr. Garang IVF and Endoscopy Institute, where participant safety is our primary clinical priority.
Clinical Eligibility for Oocyte Donation
To ensure participant safety and optimal clinical outcomes, all potential donors must meet the following Standardized Clinical Benchmarks:
- Biological Age Alignment: Candidates must be between 20 and 30 years of age to ensure optimal oocyte viability and response to clinical protocols
- Genomic Stability: Candidates must have a documented medical history free of hereditary or genomic disorders, verified through advanced screening.
- Lifestyle & Biochemical Profile: Candidates must be non-smokers and maintain a biochemical profile free from nicotine and controlled substances.
- Biometric Optimization (BMI): Candidates should maintain a Body Mass Index (BMI) within the clinically recommended range (18.5 – 25) to ensure a safe response to hormonal stimulation and sedation.
The Clinical Oocyte Donation Pathway.
Following the decision to participate in the donation program, candidates enter a structured multi-phase clinical protocol. This framework is designed to prioritize participant safety, informed consent, and medical oversight at every stage of the facilitation
Phases of the Clinical Pathway:
Initial briefing on the legal and ethical framework of donation.
Advanced diagnostic, genomic, and pathology screening at the Dr. Garang IVF and Endoscopy Institute.
A monitored pharmaceutical protocol to facilitate oocyte development.
A standardized clinical intervention performed under professional sedation.
Mandatory clinical follow-up to ensure optimal recovery and long-term well-being
Phases of the Clinical Pathway, ctn'd.
The enrollment process begins with a structured preliminary screening assessment to determine initial eligibility. Following successful intake, candidates proceed to a multi-phase vetting protocol at the Dr. Garang IVF and Endoscopy Institute, which includes:
- Diagnostic Pathology: Comprehensive blood chemistry and infectious disease screening.
- Genomic Analysis: Advanced hereditary and chromosomal evaluation.
- Psychosocial Welfare Assessment: A professional evaluation of donor suitability, motivation, and informed consent.
Upon successful completion of the clinical vetting process, donor profiles are integrated into our secure participant registry. We facilitate a structured phenotypic alignment process, allowing intended parents to identify donors whose physical characteristics and multi-generational health backgrounds align with their specific requirements. This selection phase is managed within a confidential and ethical framework, ensuring that all alignments are based on verified clinical and biographical data.
Following alignment with a case coordination plan, the donor enters a prescribed clinical protocol to facilitate controlled ovarian stimulation. This phase is managed by the medical specialists at the Dr. Garang IVF and Endoscopy Institute, utilizing evidence-based pharmaceutical protocols to optimize oocyte development.
The stimulation period is paired with rigorous diagnostic oversight, including serial ultrasound evaluations and endocrine blood analysis, to ensure a precise and safe response to the clinical protocol
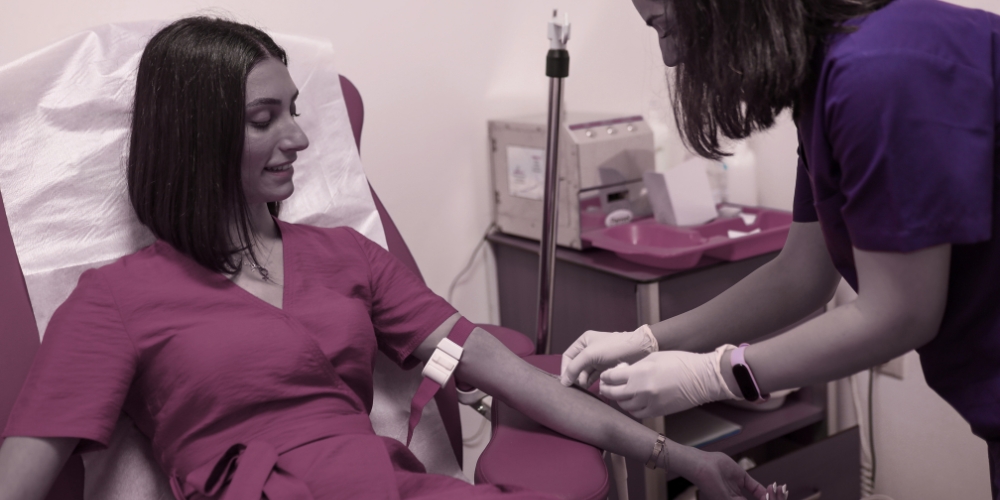
At the conclusion of the stimulation cycle, oocytes are collected via a standardized ultrasound-guided aspiration protocol. This clinical intervention is performed under professional sedation at the Dr. Garang IVF and Endoscopy Institute, ensuring a sterile, safe, and medically supervised environment. The process is designed to be efficient and minimally invasive, prioritizing participant safety and comfort through continuous monitoring by licensed clinical staff.
Following the oocyte retrieval, donors enter a structured post-procedural recovery phase. This phase is managed by the clinical team at the Dr. Garang IVF and Endoscopy Institute and includes:
- Immediate Clinical Observation: Monitoring of vital signs and physical recovery immediately following the intervention.
- Scheduled Follow-up Evaluations: Mandatory clinical assessments to confirm physiological stabilization and ensure long-term health.
- Psychosocial Welfare Support: Access to specialized counseling and administrative support to facilitate a comprehensive recovery journey.
Initiate Your Clinical Enrollment Inquiry
If you meet the standardized eligibility criteria and are prepared to contribute to the advancement of reproductive health, we invite you to begin our structured intake process.
Submit Your Preliminary Assessment
Contact Uzazi Mwelekeo Agency today to receive a comprehensive briefing on our donor welfare protocols and the clinical vetting framework at the Dr. Garang IVF and Endoscopy Institute. Our coordination team is available to provide transparent guidance on the ethical and medical standards of our program.
Clinical Diagnostic Intake & Preliminary Vetting
To maintain the highest standards of clinical safety and procedural integrity, all potential participants are required to complete a structured diagnostic intake assessment. This comprehensive questionnaire serves as the initial phase of our clinical vetting protocol, facilitating an in-depth review of your medical history, genomic profile, and biometric suitability.
This data-driven approach, managed in coordination with the Dr. Garang IVF and Endoscopy Institute, ensures that all participants meet the rigorous clinical benchmarks required for safe and ethical oocyte facilitationm
The Clinical Intake Dossier: Key Components
The assessment is divided into four primary diagnostic modules designed to establish a comprehensive donor profile:
This module establishes baseline physical health and lifestyle compatibility with clinical protocols.
- Biometric Data: Height, weight, and verified Body Mass Index (BMI).
- Lifestyle Indicators: Documentation of nicotine, alcohol, and substance use.
- Physical Characteristics: Detailed phenotypic data including eye color, hair texture, and complexion for alignment purposes.
A deep dive into the participant's clinical background to ensure procedural safety.
- Reproductive History: Menstrual cycle regularity and any previous pregnancy or donation outcomes.
- Surgical & Pathology Records: Documentation of past medical interventions and chronic conditions.
- Pharmacological History: Current medications or hormonal treatments that may interfere with stimulation protocols
To mitigate hereditary risks, we collect data on the biological family's healthhistory.
- Hereditary Risk Assessment: Screening for documented genetic disorders across two generations (parents and grandparents).
- Congenital History: Identification of any history of heart disease, cancer, or metabolic disorders within the biological lineage.
This module ensures the donor is making an informed, stable, and autonomous decision.
- Educational Background: Verification of academic achievements and specialized skills.
- Motivation & Social History: Assessment of the donor’s understanding of the bioethical implications of gamete donation.
- Mental Health Screening: Personal and family history regarding psychological well-being.
Administrative Review & Clinical Integration Milestones
Following the submission of the Diagnostic Intake Dossier, the following standardized review protocols are initiated to ensure clinical and regulatory compliance
- Phase 1: Clinical Board Review
The medical board at the Dr. Garang IVF and Endoscopy Institute conducts a comprehensive audit of the submitted health and genomic data to determine preliminary eligibility based on international ART benchmarks. - Phase 2: Advanced Clinical Vetting
Qualified candidates are invited for secondary on-site screening, including specialized diagnostic pathology, endocrine profiling, and a psychosocial welfare assessment. - Phase 3: Integration into the Secure Donor Registry
Upon clinical approval, the donor's phenotypic and medical profile is integrated into our secure, encrypted registry. This allows for precise alignment with intended parents under a strictly confidential and ethical framework
Clinical Diagnostic Screening (Oocyte Donation)
The medical vetting phase is a high-precision diagnostic protocol conducted at the Dr. Garang IVF and Endoscopy Institute to ensure physiological readiness.
- Endocrine & Ovarian Reserve AssessmentAdvanced blood analysis for AMH (Anti-Müllerian Hormone), FSH, and Estradiol levels to determine clinical response probability.
- Infectious Disease PathologyComprehensive screening for HIV, Hepatitis B/C, Syphilis, and other communicable pathogens in accordance with international health regulations.
- Genomic & Karyotype AnalysisEvaluation of chromosomal stability and screening for over 300+ recessive genetic conditions to mitigate hereditary transmission risks.
- Transvaginal Ultrasound (Antral Follicle Count)Visual diagnostic imaging to assess ovarian health and oocyte development potential.
- Participant SafetyIdentifies any underlying conditions that could increase the risk of Ovarian Hyperstimulation Syndrome (OHSS).
- Clinical EfficacyEnsures that the donor oocytes have the highest biological probability of successful fertilization and embryo development.Protects the intended parents and baby
- Regulatory ComplianceSatisfies the legal and medical requirements for international gamete facilitation.
This phase ensures that the donor is making an informed, autonomous, and psychologically stable decision.
Clinical Psychosocial Interview: A structured dialogue with a mental health professional to discuss the long-term implications of donation
- Cognitive & Motivation AssessmentReview of the donor’s understanding of the legal framework, anonymity (or lack thereof), and the ethical nature of the contribution.
- Social Support ReviewEvaluation of the donor’s external support system to ensure they have the necessary environment for a successful recovery.
- Informed Consent Integrity: Confirms the participant fully understands the clinical, legal, and emotional complexities of the process.
- Long-term Well-being: Minimizes the risk of post-procedural emotional distress and ensures a positive psychological outcome for the donor.
- Ethical Standard Adherence: Aligns with WHO and ESHRE guidelines regarding the welfare of human gamete donors.
Regulatory Compliance & Contractual Framework
Following successful clinical and psychosocial vetting, participants enter the Regulatory Compliance Phase. This framework is designed to establish a transparent legal foundation, ensuring that the rights and obligations of the donor and the intended parents are clearly defined under the relevant jurisdiction.
Core Components of the Regulatory Protocol
A structured legal instrument is prepared, outlining the specific parameters of the oocyte donation, including confidentiality, future contact protocols, and the permanent relinquishment of parental rights.
To ensure Informed Consent and autonomy, the donor is provided with access to independent legal counsel. This ensures a comprehensive understanding of the bioethical and legal implications of the agreement.
The formal signing of the agreement serves as the final administrative milestone, providing a legally sound foundation for the initiation of Controlled Ovarian Stimulation and oocyte retrieval.
This step protects everyone involved, ensuring a smooth and legally sound donation process. Once completed, you will be cleared to begin ovarian stimulation and egg retrieval
The Clinical Oocyte Facilitation Protocol
Participation in the oocyte donation program is a structured medical contribution conducted under the highest standards of participant safety. Our coordination framework ensures that every phase of the procedure is managed by licensed reproductive specialists at the Dr. Garang IVF and Endoscopy Institute.
Under the direction of a lead clinician, the donor begins a prescribed pharmaceutical protocol. This phase utilizes evidence-based medications to facilitate the synchronized development of multiple oocytes, ensuring a high probability of clinical success.
Throughout the stimulation phase, the donor undergoes serial ultrasound imaging and endocrine blood analysis. This rigorous monitoring ensures a precise response to the protocol and allows for real-time adjustments to maintain donor health and oocyte viability.
The retrieval is a standardized, minimally invasive clinical intervention performed under professional sedation. Using advanced ultrasound guidance, oocytes are aspirated in a sterile environment, prioritizing participant safety and procedural efficiency.
Following the intervention, donors remain under immediate clinical observation. A structured recovery protocol includes mandatory follow-up assessments to confirm physiological stabilization and ensure long-term well-being.
